Cell-based screening for identification of novel vanadium complexes with multidirectional activity relative to cells associated with metabolic disorders
DOI:
https://doi.org/10.5604/01.3001.0013.1047Słowa kluczowe:
vanadium complexes, cell-based screening, anti-diabetic activityAbstrakt
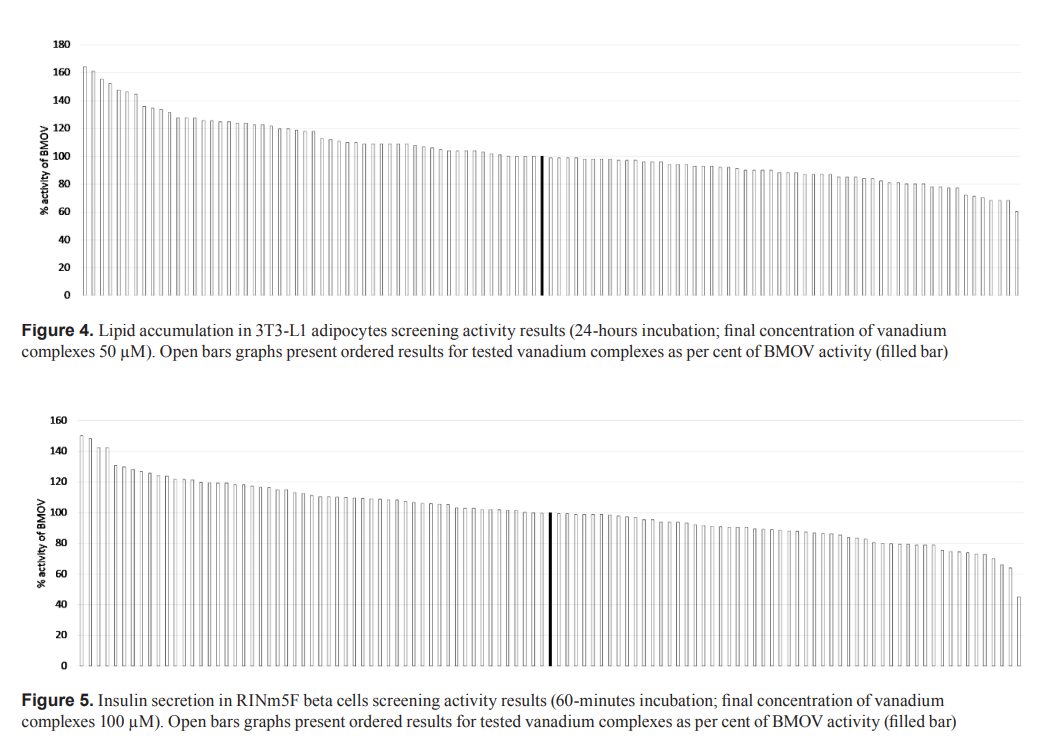
In this study, 110 newly synthesized vanadium complexes from different structural groups were screened in three cell-based models representing the main target tissues for anti-diabetic drugs. In glucose utilization in C2C12 myocyte experiments, 93% of vanadium complexes were shown to have equal or greater activity than bis(maltolato)oxovanadium(IV) (BMOV), the methyl analog of bis(ethylmaltolato)oxovanadium(IV) (BEOV) which has been tested in clinical trials. Moreover, 49% and 50% of these complexes were shown to have equal or greater activity than BMOV in lipid accumulation in 3T3-L1 adipocytes and insulin secretion in RINm5F beta cell experiments, respectively. These results were the basis for the selection of compounds for the subsequent steps in the characterization of anti-diabetic properties. This study provides strong support for the application of screening cell-based assays with a phenotypic approach for the discovery of novel anti-diabetic drugs from the vanadium complex class. This is especially desirable due to the multiple and not fully defined mechanisms of action vanadium compounds.
Statystyka pobrań
Bibliografia
Global Health Estimates 2016: Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2016. Geneva, World Health Organization; 2018, https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html Google Scholar
Miller BR, Nguyen H, Hu CJ, Lin C, Nguyen QT. New and emerging drugs and targets for type 2 diabetes: reviewing the evidence. American Health & Drug Benefits. 2014;7(8):452-463. Google Scholar
Alkhouri N, Poordad F, Lawitz E. Management of nonalcoholic fatty liver disease: Lessons learned from type 2 diabetes. Hepatology Communications. 2018 ;2(7):778-785. Google Scholar
Thompson KH, Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. Journal of Inorganic Biochemistry. 2006;100(12):1925-1935. Google Scholar
Rehder D. The role of vanadium in biology. Metallomics. 2015;7(5):730-742. Google Scholar
Rehder D. The future of/for vanadium. Dalton Transactions.2013;42(33):11749-61. Google Scholar
Shechter Y, Li J, Meyerovitch J, Gefel D, Bruck R, Elberg G, Miller DS, Shisheva A. Insulin-like actions of vanadate are mediated in an insulin-receptor-independent manner via non-receptor protein tyrosine kinases and protein phosphotyrosine phosphatases. Molecular and Cellular Biochemistry. 1995;153(1-2):39-47. Google Scholar
Venkatesan N, Avidan A, Davidson MB. Antidiabetic action of vanadyl in rats independent of in vivo insulin-receptor kinase activity. Diabetes. 1991;40(4):492-498. Google Scholar
Scior T, Guevara-Garcia JA, Do QT, Bernard P, Laufer S. Why Antidiabetic Vanadium Complexes are Not in the Pipeline of “Big Pharma” Drug Research? A Critical Review. Current Medicinal Chemistry. 2016;23(25):2874-2891. Google Scholar
Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World Journal of Diabetes. 2016;7(17):354-395. Google Scholar
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149. Google Scholar
Song R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care. 2016;39(2):187-189. Google Scholar
Abrahamson MJ. Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? Yes, they continue to serve us well! Diabetes Care. 2015;38(1):166-169. Google Scholar
Patil PD, Mahajan UB, Patil KR, Chaudhari S, Patil CR, Agrawal YO, Ojha S, Goyal SN. Past and current perspective on new therapeutic targets for Type-II diabetes. Drug Design, Development and Therapy. 2017;11:1567-1583. Google Scholar
Makinen MW, Salehitazangi M. The Structural Basis of Action of Vanadyl (VO2+) Chelates in Cells. Coordination Chemistry Reviews. 2014;279:1-22. Google Scholar
Wu Y, Huang M, Zhao P, Yang X. Vanadyl acetylacetonate upregulates PPARγ and adiponectin expression in differentiated rat adipocytes. Journal of Biological Inorganic Chemistry. 2013;18(6):623-631. Google Scholar
Niu X, Xiao R, Wang N, Wang Z, Zhang Y, Xia Q, Yang X. The Molecular Mechanisms and Rational Design of Anti-Diabetic Vanadium Compounds. Current Topics in Medicinal Chemistry. 2016;16(8):811-822. Google Scholar
Missaoui S, Ben Rhouma K, Yacoubi MT, Sakly M, Tebourbi O. Vanadyl sulfate treatment stimulates proliferation and regeneration of beta cells in pancreatic islets. Journal of Diabetes Research. 2014;2014:540242. Google Scholar
Gao Z, Zhang C, Yu S, Yang X, Wang K. Vanadyl bisacetylacetonate protects β cells from palmitate-induced cell death through the unfolded protein response pathway. Journal of Biological Inorganic Chemistry. 2011;16(5):789-798. Google Scholar
Zheng W, Thorne N, McKew JC. Phenotypic screens as a renewed approach for drug discovery. Drug Discovery Today. 2013;18(21-22):1067-1073. Google Scholar
Wagner BK. The resurgence of phenotypic screening in drug discovery and development. Expert Opinion on Drug Discovery. 2016;11(2):121-125. Google Scholar
Hou WL, Yin J, Alimujiang M, Yu XY, Ai LG, Bao YQ, Liu F, Jia WP. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. Journal of Cellular and Molecular Medicine. 2018;22(2):1316-1328. Google Scholar
Sun B, Zhong Z, Wang F, Xu J, Xu F, Kong W, Ling Z, Shu N, Li Y, Wu T, Zhang M, Zhu L, Liu X, Liu L. Atorvastatin impaired glucose metabolism in C2C12 cells partly via inhibiting cholesterol-dependent glucose transporter 4 translocation. Biochemical Pharmacology. 2018;150:108-119. Google Scholar
Zhang L, Huang Y, Liu F, Zhang F, Ding W. Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. Journal of Inorganic Biochemistry. 2016;162:1-8. Google Scholar
Gundhla IZ, Walmsley RS, Ugirinema V, Mnonopi NO, Hosten E, Betz R, Frost CL, Tshentu ZR. pH-metric chemical speciation modeling and studies of in vitro antidiabetic effects of bis[(imidazolyl)carboxylato]oxidovanadium(IV) complexes. Journal of Inorganic Biochemistry. 2015;145:11-18. Google Scholar
Zeng XY, Zhou X, Xu J, Chan SM, Xue CL, Molero JC Ye JM. Screening for the efficacy on lipid accumulation in 3T3-L1 cells is an effective tool for the identification of new anti-diabetic compounds. Biochemical Pharmacology. 2012;84(6):830-837. Google Scholar
Liu JC, Yu Y, Wang G, Wang K, Yang XG. Bis(acetylacetonato)-oxovanadium(iv), bis(maltolato)-oxovanadium(iv) and sodium metavanadate induce antilipolytic effects by regulating hormone-sensitive lipase and perilipin via activation of Akt. Metallomics. 2013 ;5(7):813-820. Google Scholar
Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010;27(2):105-113. Google Scholar
Rumora L, Hadzija M, Maysinger D, Zanić-Grubisić T. Positive regulation of ERK activation and MKP-1 expression by peroxovanadium complex bpV (phen). Cell Biology and Toxicology. 2004;20(5):293-301. Google Scholar
Leney SE, Tavaré JM. The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. Journal of Endocrinology. 2009;203(1):1-18. Google Scholar
Vogel HG, Editor. Drug Discovery and Evaluation: Pharmacological Assays. Springer-Verlag, 2013. Google Scholar
Burns SM, Vetere A, Walpita D, Dančík V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metabolism. 2015;21(1):126-137. Google Scholar
Porte D, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50 Suppl 1:S160-S163. Google Scholar
Thompson KH, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C. Vanadium treatment of type 2 diabetes: a view to the future. Journal of Inorganic Biochemistry. 2009 ;103(4):554-558. Google Scholar
Conconi MT, DeCarlo E, Vigolo S, Grandi C, Bandoli G, Sicolo N, Tamagno G, Parnigotto PP, Nussdorfer GG. Effects of some vanadyl coordination compounds on the in vitro insulin release from rat pancreatic islets. Horm Metab Res. 2003 Jul;35(7):402-406. Google Scholar
Yu-Bing Sun, Qing Xie, Wei Li, Yi Ding, Yu-Ting Ye. Synthesis, Crystal Structures, and Insulin Enhancement of Vanadium(V) Complexes Derived From 2-Bromo-N’-(2-hydroxybenzylidene)benzohydrazide, Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 46:11, 1613-1617. Google Scholar
Pobrania
Opublikowane
Jak cytować
Numer
Dział
Licencja
Prawa autorskie (c) 2019 Państwowa Wyższa Szkoła Zawodowa w Tarnowie & Autorzy

Utwór dostępny jest na licencji Creative Commons Uznanie autorstwa – Użycie niekomercyjne 4.0 Międzynarodowe.



