Tridentate hydrazido-hydrazones vanadium complexes
Synthesis, properties and biological activity
DOI:
https://doi.org/10.5604/01.3001.0013.1485Keywords:
vanadium, complex, Schiff base, structure, hydrazides, biological activityAbstract
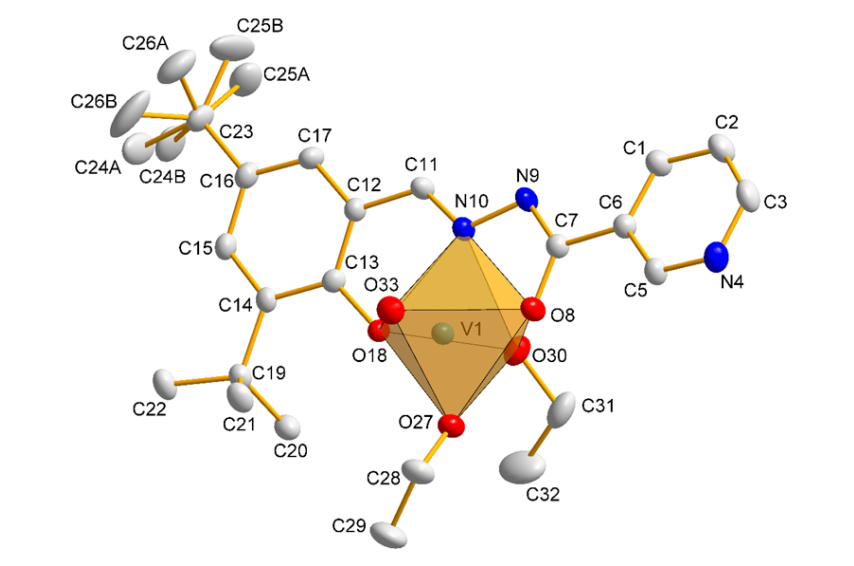
Nine new vanadium complexes, with tridentate Schiff base ligand based on 3,5-di-tert-butyl-2-hydroxybenzaldehyde and different hydrazides, are described and characterized. The X-ray crystal structure of complex 8 shows distorted octahedral geometry of vanadium, with ONO ligand in equatorial position. The tridentate Schiff base ligand forms six membered and five-membered chelate rings at the V(V) acceptor center, with the corresponding bite angles being 82.97(9)˚ and 74.48(9)˚. The molecules are gathered by means of intermolecular O-H∙∙∙N hydrogen bond and layered by π∙∙∙π interactions involving the pyridine and phenolate rings. Such interactions expand the structure along the crystallographic a axis. The complexes were characterized by the elemental analyses, IR, UV-Vis, EPR spectroscopy, cyclic voltammetry, thermogravimetry and magnetic susceptibility measurements. The stabilization role of co-ligands is discussed. The cytotoxicity versus HepG2 hepatocytes and inhibition of human recombinant PTP1B was studied.
Downloads
References
Rehder D. Implications of vanadium in technical applications and pharmaceutical issues. Inorganica Chimica Acta. 2017; 455:378-389. Google Scholar
Azza AA. Synthesis and spectroscopic studies on ternary bis-Schiff-base complexes having oxygen and/or nitrogen donors. Journal of Coordination Chemistry. 2006; 59:157-176. Google Scholar
Pessoa JC, Etcheverry S, Gambino D. Vanadium compounds in medicine. Coordination Chemistry Review. 2015; 301-302:24-48. Google Scholar
Dhar DN, Taploo CL. Schiff-bases and their applications. Journal of Scientific and Industrial Research. 1982; 41:501-506. Google Scholar
Jia Y, Li J. Molecular Assembly of Schiff Base Interactions: Construction and Application. Chemical Review. 2015; 115:1597-1621. Google Scholar
Przybylski P, Huczynski A, Pyta K, Brzezinski B, Bartl F. Biological properties of Schiff bases and azo derivatives of phenols. Current Organic Chemistry. 2009; 13:124-148. Google Scholar
Ebrahimipour SY, Sheikhshoaie I, Kautz AC, Ameri M, Pasban-Alibadi F, Rudbari HA, Bruno G, Janiak Ch. Mono-and dioxido-vanadium (V) complexes of a tridentate ONO Schiff base ligand: Synthesis, spectral characterization, X-ray crystal structure, and anticancer activity. Polyhedron. 2015; 93:99-105. Google Scholar
Zamarin D. Vanadium: A Panacea for Resistance to Oncolytic Immunotherapy? Molecular Therapy. 2018; 26:9-12. Google Scholar
Zabierowski P, Szklarzewicz J, Gryboś R, Modryl B, Nitek W. Assemblies of salen-type oxidovanadium (IV) complexes: substituent effects and in vitro protein tyrosine phosphatase inhibition. Dalton Transactions. 2014; 43:17044-17053. Google Scholar
Gryboś R, Szklarzewicz J, Jurowska A, Hodorowicz M. Properties, structure and stability of V (IV) hydrazide Schiff base ligand complex. Journal of Molecular Structure. 2018;1171:880-887. Google Scholar
Ma L, Lu L, Zhu M, Wang Q, Gao F, Yuan C, Wu Y, Xing S, Fu X, Mei Y, Gao X. Dinuclear copper complexes of organic claw: potent inhibition of protein tyrosine phosphatases. Journal of Inorganic Biochemistry. 2011; 105:1138-1147. Google Scholar
Wang Q, Zhu M, Lu L, Yuan C, Xing S, Fu X. Potent inhibition of protein tyrosine phosphatases by quinquedentate binuclear copper complexes: synthesis, characterization and biological activities. Dalton Transactions. 2011; 40:12926-12934. Google Scholar
Lu L, Zhu M. Protein tyrosine phosphatase inhibition by metals and metal complexes. Antioxidants and Redox Signaling. 2014; 20:2210-2224. Google Scholar
Thompson KH, Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. Journal of Inorganic Biochemistry. 2006; 100:1925-1935. Google Scholar
Domingo JL, Gomez M, Sanchez DJ, Llobet JM, Keen CL. Toxicology of vanadium compounds in diabetic rats: the action of chelating agents on vanadium accumulation. Molecular and Cellular Biochemistry. 1995; 153:233–240. Google Scholar
Domingo JL. Vanadium and tungsten derivatives as antidiabetic agents. Biological Trace Element Research. 2002; 88:97–112. Google Scholar
Gruzewska K, Michno A, Pawelczyk T, Bielarczyk H. Essentiality and toxicity of vanadium supplements in health and pathology. Journal of Physiology and Pharmacology. 2014; 65:603-611. Google Scholar
Evangelou AM. Vanadium in cancer treatment. Critical Reviews in Oncology/Hematology. 2002; 42:249-265. Google Scholar
Llobet JM, Domingo JL. Acute toxicity of vanadium compounds in rats and mice. Toxicology Letters. 1984; 23:227-231. Google Scholar
Srivastava AK. Anti-diabetic and toxic effects of vanadium compounds. Molecular and Cellular Biochemistry. 2000; 206:177-182. Google Scholar
Reul BA, Amin SS, Buchet JP, Ongemba LN, Crans DC, Brichard SM. Br. Effects of vanadium complexes with organic ligands on glucose metabolism: a comparison study in diabetic rats. British Journal of Pharmacology. 1999; 126:467-477. Google Scholar
Fujimoto S, Fujii K, Yasui H, Matsushita R, Takada J, Sakurai H. Long-term acting and orally active vanadyl-methylpicolinate complex with hypoglycemic activity in streptozotocin-induced diabetic rats. Journal of Clinical Biochemistry and Nutrition. 1997; 23:113–129. Google Scholar
Halevas E, Tsave O, Yavropoulou MP, Hatzidimitriou A, Yovos JG, Psycharis V, Gabriel C, Salifoglou A. Design, synthesis and characterization of novel binary V (V)-Schiff base materials linked with insulin-mimetic vanadium-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Structure–function correlations at the molecular level. Journal of Inorganic Biochemistry. 2015; 147:99-115. Google Scholar
Sugiyama H, Matsugo S, Misu H, Takamura T, Kaneko S, Kanatani Y, Kaido M, Mihara C, Abeywardana N, Sakai A, Sato K, Miyashita Y, Kanamori K. Regulation of the physiological effects of peroxidovanadium (V) complexes by the electronic nature of ligands. Journal of Inorganic Biochemistry. 2013; 121:66-76. Google Scholar
Dümmling S, Eichhorn E, Schneider S, Speiser B, Würde M. Recycling of the Supporting Electrolyte Tetra(n-butyl)ammonium Hexafluorophosphate from Used Electrolyte Solutions. Current Separations. 1996; 5:53-56. Google Scholar
Gryboś R, Paciorek P, Szklarzewicz J, Matoga D, Zabierowski P, Kazek G. Novel vanadyl complexes of acetoacetanilide Synthesis, characterization and inhibition of proteintyrosine phosphatase. Polyhedron. 2013;49:100-104. Google Scholar
Sutradhar M, Martins LM, Carabineiro SA, Guedes da Silva MFC, Buijnsters JG, Figueiredo JL, Pombeiro AJL. Oxidovanadium (V) Complexes Anchored on Carbon Materials as Catalysts for the Oxidation of 1-Phenylethanol. Chem. Cat. Chem. 2016;8:2254-2266. Google Scholar
Szklarzewicz J, Jurowska A, Hodorowicz M, Gryboś R, Matoga D. Role of co-ligand and solvent on properties of V(IV) oxido complexes with ONO Schiff bases. Journal of Molecular Structure. 2019; 1180:839-848. Google Scholar
Sheldrick GM. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallographica Section A Foundations and Advances. 2015; 71:3-8. Google Scholar
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallographica Section C Structural Chemistry. 2015; 71:3-8. Google Scholar
Brandenburg K, Putz H. DIAMOND. Crystal Impact GbR, Bonn, Germany. 2005. Google Scholar
Tsiamis C, Voulgaropoulos B, Charistos D, Voutsas GP, Kavounis C. Ligand reactivity, substituent effects and solvent interactions in the spectra and structure of some oxovanadium (V) chelates with Schiff bases. Proof for dimeric species. Polyhedron. 2000; 19:2003-2010. Google Scholar
Diamantis AA, Frederiksen JM, Salam MA, Snow MR, Tiekink ERT. Structures of 2 Vanadium (V) Complexes with Tridentate Ligands. Australian Journal of Chemistry. 1986; 39:1081-1088. Google Scholar
Wang W, Wang X, Liu HX, Tan MY. Synthesis, characterization and crystal structure of an oxovanadium (V) complex with the Schiff base N-benzoylacetone-M-chlorobenzoylhydrazone. Journal of Coordination Chemistry 1995; 36:49-55. Google Scholar
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2019 University of Applied Sciences in Tarnow, Poland & Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



