Synthesis, structure and properties of V(IV) complex with N’-[(E)-(2,3-dihydroxyphenyl)metylidene]- -2-phenylacetohydrazide
DOI:
https://doi.org/10.5604/01.3001.0013.1484Keywords:
vanadium, complex, Schiff base, structure, 2,3-dihydroxybenzaldehyde, phenylacetic hydrazideAbstract
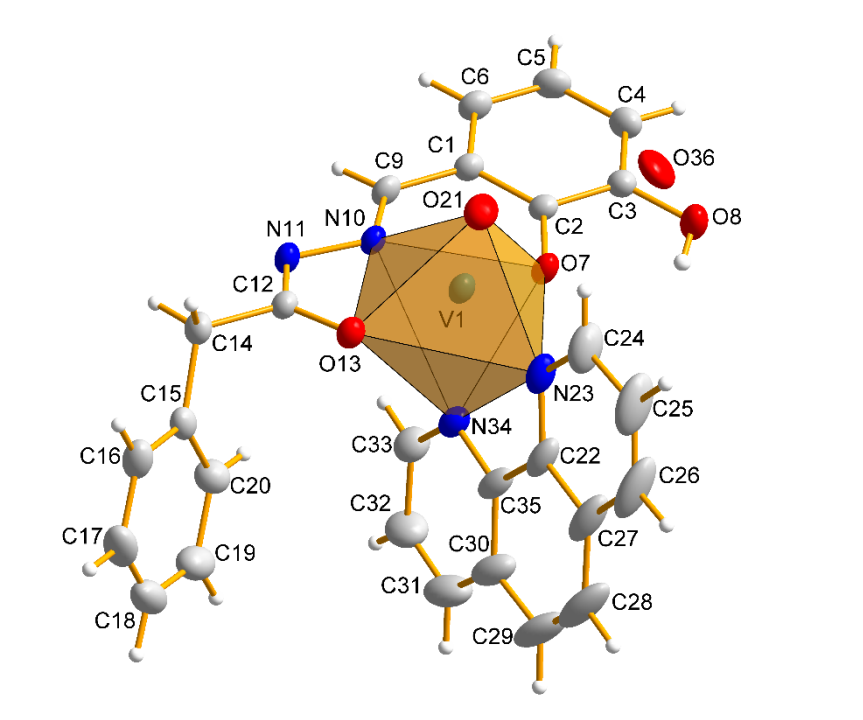
The synthesis and physicochemical properties of new vanadium(IV) complex of formula [VO(L)(phen)] is described. The L denotes ONO tridentate Schiff base derived from 2,3-dihydroxybenzaldehyde and phenylacetic hydrazide, while phen = 1,10-phenanthroline used as a co-ligand to stabilize the V(IV) oxidation state. The single crystal X-Ray crystal structure indicates on octahedral geometry of vanadium centre, with 1,10-phenanthroline nitrogen trans to the V=O bond. The complex crystalizes in a monoclinic P21/c space group, very unusual is that only one isomer is present in the crystal structure. The structure is stabilized by very weak hydrogen bonds and H···π and π···π interactions. The phenyl ring of hydrazide is strongly curved from ONO ligand plane by 70.95˚. The spectroscopic characterization (IR, UV-Vis) as well as the cyclic voltammetry measurements are presented and discussed.
Downloads
References
Mukherjee T, Costa Pessoa J, Kumar A, Sarkar AR. Oxidovanadium (IV) Schiff base complex derived from vitamin B6: synthesis, characterization, and insulin enhancing properties. Inorganic Chemistry. 2011; 50:4349-4361. Google Scholar
Holder AA, Taylor P, Magnusen AR, Moffett ET, Meyer K, Hong Y, Ramsdale SE, Gordon M, Stubbs J, Seymour LA, Acharya D, Weber RT, Smith PF, Dismukes GC, Ji P, Menocal L, Bai F, Williams JL, Cropek DM, Jarrett WL. Preliminary anti-cancer photodynamic therapeutic in vitro studies with mixed-metal binuclear ruthenium (II)–vanadium (IV) complexes. Dalton Transactions. 2013; 42:11881-11899. Google Scholar
León IE, Parajón-Costa BS, Franca CA, Etcheverry SB, Baran EJ. A New Oxidovanadium (IV) Complex of Oxodiacetic Acid and dppz: Spectroscopic and DFT Study. Antitumor Action on MG-63 Human Osteosarcoma Cell Line. Biological Trace Element Research. 2015; 164:198-204. Google Scholar
Abaszadeh M, Seifi M, Ebrahimipour SY. Two ligand oxidio-vanadium (IV) complexes as novel efficient catalysts in multicomponent reactions for synthesis of tetrahydrobenzopyran derivatives. Bulletin of the Chemical Society of Ethiopia. 2016; 30:253-262. Google Scholar
Bikas R, Shahmoradi E, Noshiranzadeh N, Emami M, Reinoso S. The effects of halogen substituents on the catalytic oxidation of benzyl-alcohols in the presence of dinuclear oxidovanadium (IV) complex. Inorganica Chimica Acta. 2017; 466:100-109. Google Scholar
Sakurai H, Fujii K, Watanabe H, Tamura H. Orally active and long-term acting insulin-mimetic vanadyl complex: bis (picolinato) oxovanadium (IV). Biochemical and Biophysical Research Communications. 1995; 214:1095-1101. Google Scholar
Melchior M, Thompson KH, Jong JM, Rettig SJ, Shuter E, Yuen VG, Zhou Y, McNeill JH, Orvig C. Vanadium complexes as insulin mimetic agents: coordination chemistry and in vivo studies of oxovanadium (IV) and dioxovanadate (V) complexes formed from naturally occurring chelating oxazolinate, thiazolinate, or picolinate units. Inorganic Chemistry. 1999; 38:2288-2293. Google Scholar
Rehder D, Pessoa JC, Geraldes CF, Castro MM, Kabanos T, Kiss T, Meier B, Micera G, Pettersson L, Rangel M, Salifoglou A, Turel I, Wang D. In vitro study of the insulin-mimetic behaviour of vanadium (IV, V) coordination compounds. JBIC Journal of Biological Inorganic Chemistry. 2002; 7:384-396. Google Scholar
Halevas E, Tsave O, Yavropoulou MP, Hatzidimitriou A, Yovos JG, Psycharis V, Gabriel C, Salifoglou A. Design, synthesis and characterization of novel binary V(V)-Schiff base materials linked with insulin-mimetic vanadium-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Structure–function correlations at the molecular level. Journal of Inorganic Biochemistry. 2015; 147:99-115. Google Scholar
Li SY, Zhai WQ, Li ZW, Li A, Jiang YM, Li W. Synthesis, Crystal Structures and Insulin-Enhancing Activity of Vanadium (V) Complexes with Hydrazone Ligands. Russian Journal of Coordination Chemistry. 2018; 44:701-706. Google Scholar
Yuen VG, Orvig C, McNeill JH. Glucose-lowering effects of a new organic vanadium complex, bis (maltolato) oxovanadium (IV). Canadian Journal of Physiology and Pharmacology. 1993; 71:263-269. Google Scholar
Cong XQ, Piao MH, Li Y, Xie L, Liu Y. Bis (maltolato) oxovanadium (IV)(BMOV) attenuates apoptosis in high glucose-treated cardiac cells and diabetic rat hearts by regulating the unfolded protein responses (UPRs). Biological Trace Element Research. 2016; 173:390-398. Google Scholar
Wang J, Yuen VG, McNeill JH. Effect of vanadium on insulin and leptin in Zucker diabetic fatty rats. Molecular and Cellular Biochemistry. 2001; 218:93-96. Google Scholar
Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD. The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone. 2016; 38:368-377. Google Scholar
Jakusch T, Kiss T. In vitro study of the antidiabetic behawior of vanadium compounds. Coordination Chemistry Reviews. 2017; 351:118-126. Google Scholar
Patra D, Paul S, Sepay N, Kundu R, Ghosh T. Structure-activity relationship on DNA binding and anticancer activities of a family of mixed-ligand oxidovanadium (V) hydrazone complexes. Journal of Biomolecular Structure and Dynamics. 2017; 13:1-13. Google Scholar
Torabi S, Mohammadi M, Shirvani M. Antidiabetic, Antioxidant, Antibacterial, and Antifungal Activities of Vanadyl Schiff Base Complexes. Trends in Pharmaceutical Sciences. 2018; 4:87-94. Google Scholar
Farzanfar J, Ghasemi K, Rezvani AR, Delarami HS, Ebrahimi A, Hosseinpoor H, Eskandari A, Rudbari HA, Bruno G. Synthesis, characterization, X-ray crystal structure, DFT calculation and antibacterial activities of new vanadium (IV, V) complexes containing chelidamic acid and novel thiourea derivatives. Journal of Inorganic Biochemistry. 2015; 147:54-64. Google Scholar
Chen XF, Wang TR, Ma Z, Yu Y, Tang L, Jin LY, Sheng GH, Zhu HL. Synthesis, crystal structures and insulin-like activity of two new oxidovanadium (V) complexes with aroylhydrazones and maltol mixed-ligands. Polyhedron. 2017; 137:321-324. Google Scholar
Zhao X, Chen X, Li J, Chen J, Sheng G, Niu F, Qu D, Huo Y, Zhu H, You Z. Synthesis, structures and insulin-like activity of oxidovanadium (V) complexes with aroylhydrazones and ethylmaltol mixed-ligands. Polyhedron. 2015; 97:268-272. Google Scholar
Thompson, K.H., Lichter J, LeBel K, Scaife MC, McNeill JH, Orvig C, Vanadium treatment of type 2 diabetes: A view to the future. Journal of Inorganic Biochemistry. 2009; 103:554-558 Google Scholar
Prakash A, Adhikari D. Application of Schiff bases and their metal complexes-A Review. International Journal of ChemTech Research. 2011; 3:1891-1896. Google Scholar
Zaheer M, Shah A, Akhter Z, Qureshi R, Mirza B, Tauseef M, Bolte M. Synthesis, characterization, electrochemistry and evaluation of biological activities of some ferrocenyl Schiff bases. Applied Organometallic Chemistry. 2011; 25:61-69. Google Scholar
Hranjec M, Starčević K, Pavelić SK, Lučin P, Pavelić K, Zamola GK. Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. European Journal of Medicinal Chemistry. 2011; 46:2274-2279. Google Scholar
Dümmling S, Eichhorn E, Schneider S, Speiser B, Würde M. Recycling of the Supporting Electrolyte Tetra(n-butyl)ammonium Hexafluorophosphate from Used Electrolyte Solutions. Current Separations. 1996; 5:53-56. Google Scholar
Nonius COLLECT. Nonius BV, Delft, The Netherlands; 1998. Google Scholar
Otwinowski Z, Minor W., Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by Carter Jr CW, Sweet RM, New York: Academic Press; 1997. p. 307–326. Google Scholar
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallographica C Structural Chemistry. 2015; 71:3-8. Google Scholar
Brandenburg K, Putz H. DIAMOND. Crystal Impact GbR, Bonn, Germany; 2015. Google Scholar
Repich HH, Orysyk SI, Orysyk VV, Zborovskii YL, Melnyk AK, Trachevskyi VV, Pekhnyo VI, Vovk MV. Influence of synthesis conditions on complexation of Cu (II) with O,N,O tridentate hydrazone ligand. X-ray diffraction and spectroscopic investigations. Journal of Molecular Structure. 2017; 1146:222- 232. Google Scholar
Luo W, Meng X-G, Xiang J-F, Duan Y, Cheng G-Z, Ji Z-P. Synthesis, characterization and bioactivity of four novel trinuclear copper(II) and nickel(II) complexes with pentadentate ligands derived from N-acylsalicylhydrazide. Inorganica Chimica Acta. 2008; 361:2667-2676. Google Scholar
Wilde F, Lemmerhirt H, Emmrich T, Bednarski PJ, Link A. Microwave-assisted synthesis and evaluation of acylhydrazones as potential inhibitors of bovine glutathione peroxidase. Molecular Diversity. 2014; 18:307-322. Google Scholar
Repich HH, Orysyk SI, Orysyk VV, Zborovskii YL, Pekhnyo VI, Vovk MV. Synthesis, crystal structure and spectral characterization of the first Ag+ complex compounds involving O,N,O-coordinated N-acylhydrazones of salicylaldehyde. Journal of Molecular Structure. 2017; 1144:225-236. Google Scholar
Luo W, Wang XT, Meng X-G, Cheng G-Z, Ji Z-P. Metal coordination architectures of N-acyl-salicylhydrazides: The effect of metal ions and steric repulsion of ligands to their structures of polynuclear metal complexes. Polyhedron. 2009; 28:300-306. Google Scholar
Gryboś R, Szklarzewicz J, Jurowska A, Hodorowicz M. Properties, structure and stability of V(IV) hydrazide Schiff base ligand complex. Journal of Molecular Structure. 2018; 1171:880-887. Google Scholar
Mathew M, Carty AJ, Palenik GJ. An Unusual Complex Containing Bridging Vanadyl Groups. The Crystal Structure of N,N′-Propylenebis(salicylaldiminato)oxovanadium(IV). Journal of the American Chemical Society. 1970; 92:3197–3198. Google Scholar
Cashin B, Cunningham D, Daly P, McArdle P, Munroe M, Ní Chonchubhair N. Donor properties of the vanadyl ion: Reactions of vanadyl salicylaldimine β-ketimine and acetylacetonato complexes with groups 14 and 15 Lewis acids. Inorganic Chemistry. 2002; 41:773–782. Google Scholar
Schilt A, Taylor RC. Infra-red spectra of 1,10-phenanthroline metal complexes in the rock salt region below 2000 cm-1. Journal of Inorganic and Nuclear Chemistry. 1959; 9:211-221. Google Scholar
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2019 University of Applied Sciences in Tarnow, Poland & Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



