Charakterystyka struktury elektronowej heteropolikwasu fosforowolframowego H₃PW₁₂O₄₀ modyfikowanego kationem Fe²⁺
DOI:
https://doi.org/10.5604/01.3001.0014.7530Słowa kluczowe:
heteropolikwasy, DFT, analiza strukturalnaAbstrakt
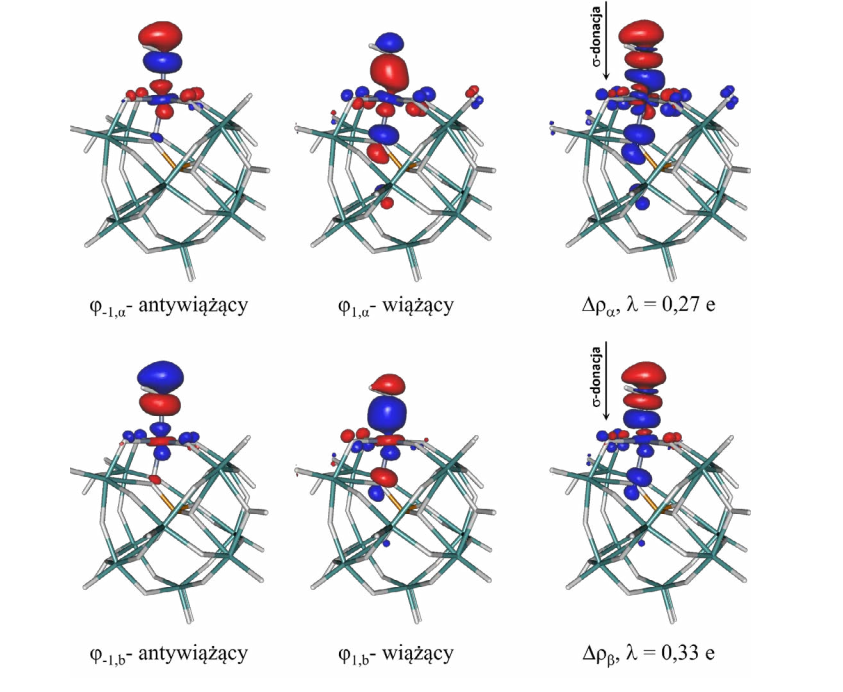
W pracy zbadano wpływ podstawienia atomu wolframu atomem żelaza w pierwszorzędowej strukturze heteropolikwasu fosforowolframowego o budowie anionu Keggina. Charakterystykę struktury elektronowej zmodyfikowanego heteropolikwasu przeprowadzono za pomocą: analizy populacyjnej NBO, całkowitych (TDOS) i parcjalnych (PDOS) widm gęstości stanów, energetyki i charakteru chemicznego orbitali granicznych (HOMO/LUMO) oraz rozmiaru przerwy wzbronionej (GAP). Dodatkowo zbadano mechanizm oddziaływania modyfikowanego kationem Fe²⁺ heteropolikwasu fosforowolframowego z cząsteczką H₂O pełniącą rolę środowiska reakcji chemicznej. W większości charakterystyk stwierdzono istotny wpływ wprowadzonego metalu przejściowego na ww. właściwości w stosunku do heteropolikwasu wyjściowego H₃PW₁₂O₄₀.
Statystyka pobrań
Bibliografia
Wei Y, Xu B, Barnes CL, Peng Z. An efficient and convenient reaction protocol to organoimido derivatives of polyoxometalates. Journal of the American Chemical Society. 2001;123(17):4083–4084. doi: https://doi.org/10.1021/ja004033q. Google Scholar
Vazylyev M, Sloboda-Rozner D, Haimov A, Maayan G, Neumann R. Strategies for oxidation catalyzed polyoxometalates at the interference of homogeneous and heterogeneous catalysis. Topics in Catalysis. 2005;34:93–99. doi: https://doi.org/10.1007/s11244-005-3793-5. Google Scholar
Shigeta S, Mori S, Kodama E, Kodama J, Takahashi K, Yamase T. Broad spectrum anti-RNA virus activities of titanium and vanadium substituted polyoxotungstates. Antiviral Research. 2003;58(3):265–271. doi: https://doi.org/10.1016/S0166-3542(03)00009-3. Google Scholar
Hierle R, Badan J, Zyss J. Growth and characterization of a new material for nonlinear optics: methyl- 3-nitro-4-pyridine-1-oxide (POM). Journal of Crystal Growth. 1984;69(2-3):545–554. doi: https://doi.org/10.1016/0022-0248(84)90366-X. Google Scholar
Qiu W, Zheng Y, Haralampides KA. Study on a novel POM-based magnetic photocatalyst: Photocatalytic degradation and magnetic separation. Chemical Engineering Journal. 2007;125(3):165–176 doi: https://doi.org/10.1016/j.cej.2006.08.025. Google Scholar
Mizuno N, Misono M. Heterogeneous catalysis. Chemical Reviews. 1998;98(1):199–218. doi: https://doi.org/10.1021/cr960401q. Google Scholar
Kozhevnikov IV. Catalysis by heteropoly acids and multicomponent poly oxometalates in liquid-phase reactions. Chemical Reviews. 1998;98(1):171–198. doi: https://doi.org/10.1021/cr960400y. Google Scholar
Okuhara T, Mizuno N, Misono M. Catalytic chemistry of heteropoly compounds. Advances in Catalysis. 1996;41:113–252. doi: https://doi.org/10.1016/S0360-0564(08)60041-3. Google Scholar
Kozhevnikov IV. Heteropoly acids and related compounds as catalysts for fine chemical synthesis. Catalysis Reviews: Science and Engineering. 1995;37(2):311–352. doi: https://doi.org/10.1080/01614949508007097. Google Scholar
Casarini D, Centi G, Jiru P, Lena V, Tvaruzkova Z. Reactivity of molybdovanadophosphoric acids: influence of the presence of vanadium in the primary and secondary structure. Journal of Catalysis. 1993;143(2):325–344. doi: https://doi.org/10.1006/jcat.1993.1280. Google Scholar
Harrup MK, Hill CL. Polyoxometalate catalysis of the aerobic oxidation of hydrogen sulfide to sulfur. Inorganic Chemistry. 1994;33(24):5448–5455. doi: https://doi.org/10.1021/ic00102a017. Google Scholar
Neumann R, Abu-Gnim C. Alkene oxidation catalyzed by a ruthenium-substituted heteropolyanion, SiRu(L)W11O39: the mechanism of the periodate-mediated oxidative cleavage. Journal of the American Chemical Society.1990;112(16):6025–6031. doi: https://doi.org/10.1021/ja00172a018. Google Scholar
Weber RS. Molecular orbital study of C-H bond breaking during the oxidative dehydrogenation of methanol catalyzed by metal oxide surfaces. Journal of Physical Chemistry. 1994;98(11):2999–3005. doi: https://doi.org/10.1021/j100062a042. Google Scholar
Zhang FQ, Zhang XM, Wu HS, Jiao H. Structural and electronic properties of hetero-transition-metal Keggin anions: a DFT study of α/β-[XW12O40]n− (X = CrVI, VV, TiIV, FeIII, CoIII, NiIII, CoII, and ZnII) relative stability. Journal of Physical Chemistry A. 2007;111(1):111, 159–166. doi: https://doi.org/10.1021/jp064732a. Google Scholar
Maestre JM, Lopez X, Bo C, Poblet J-M, Casañ-Pastor N. Electronic and magnetic properties of α-Keggin anions: a DFT study of [XM12O40]n−, (M = W, Mo; X = AlIII, SiIV, PV, FeIII, CoII, CoIII) and [SiM11VO40]m− (M = Mo and W). Journal of the American Chemical Society. 2001;123(16):3749–3758. doi: https://doi.org/10.1021/ja003563j. Google Scholar
Zonnevijlle F, Tourné CM, Tourné GF. Preparation and characterization of heteropolytungstates containing group 3a elements. Inorganic Chemistry. 1982;21(7):2742–2750. doi: https://doi.org/10.1021/ic00137a041. Google Scholar
Nakajima K, Eda K, Himeno S. Effect of the central oxoanion size on the voltammetric properties of Keggin-type [XW12O40]n− (n = 2 − 6) complexes. Inorganic Chemistry. 2010;49(11):5212–5216. doi: https://doi.org/10.1021/ic1003353. Google Scholar
Altenau JJ, Pope MT, Prados RA, So H. Models for heteropoly blues: Degrees of valence trapping in vanadium(IV)- and molybdenum(V)-substituted Keggin anions. Inorganic Chemistry. 1975;14(2):417–421. doi: https://doi.org/10.1021/ic50144a042. Google Scholar
Keita B, Nadjo L. New oxometalate-based materials for catalysis and electrocatalysis. Materials Chemistry and Physics. 1989;22(1–2):77–103. doi: https://doi.org/10.1016/0254-0584(89)90032-1. Google Scholar
Sun W, Liu H, Kong J, Xie G, Deng J. Redox electrochemistry of Keggin type iridium-substituted heteropolytungstates and their electrocatalytic activity toward the reduction of nitrite ion. Journal of Electroanalytical Chemistry. 1997;437(1–2):67–76. doi: https://doi.org/10.1016/S0022-0728(97)00356-2. Google Scholar
Rong C, Anson FC. Simplified preparations and electrochemical behavior of two chromium-substituted heteropolytungstate anions. Inorganic Chemistry. 1994;33(6):1064–1070. doi: https://doi.org/10.1021/ic00084a016. Google Scholar
Maeda K, Katano H, Osakai T, Himeno S, Saito A. Charge dependence of one-electron redox potentials of Keggin-type heteropolyoxometalate anions. Journal of Electroanalytical Chemistry. 1995;389(1–2):167–173. doi: https://doi.org/10.1016/0022-0728(95)03872-E. Google Scholar
Dong S, Xi X, Tian M. Study of the electrocatalytic reduction of nitrite with silicotungstic heteropolyanion. Journal of Electroanalytical Chemistry. 1995;385(2):227–233. doi: https://doi.org/10.1016/0022-0728(94)03770-4. Google Scholar
Turbomole V6.3 2011 adoUoKa. Forschungszentrum Karlsruhe GmbH – Turbomole GmbH saf. http://www.turbomole.com. Google Scholar
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Physical Review Letters. 1996;77(18):3865–3868. doi: https://doi.org/10.1103/PhysRevLett.77.3865. Google Scholar
Slater JC. The self-consistent field for molecular and solids. Quantum theory of molecular and solids. Vol. 4. New York: McGraw-Hill; 1974. Google Scholar
Perdew JP, Wang Y. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Physical Review B. Condensed Matter. 1992;46(20):12947–12954. doi: https://doi.org/10.1103/physrevb.46.12947. Google Scholar
Schaefer A, Horn H, Ahlrichs R. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. The Journal of Chemical Physics. 1992;97: 2571. Google Scholar
Schäfer A, Horn H, Ahlrichs R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. The Journal of Chemical Physics. 1992;97(4):2571–2577. doi: https://doi.org/10.1063/1.463096. Google Scholar
Pearson RG. Hard and soft acids and bases. Journal of the American Chemical Society. 1963;85(22):3533–3539. doi: https://doi.org/10.1021/ja00905a001. Google Scholar
Pearson RG. Hard and soft acids and bases, HSAB. Part 1: Fundamental principles. Journal of Chemical Education. 1968;45(9):581–586. doi: https://doi.org/10.1021/ed045p581. Google Scholar
Pearson RG. Hard and soft acids and bases, HSAB. Part II: Underlying theories. Journal of Chemical Education. 1968;45(10):643–648. doi: https://doi.org/10.1021/ed045p643. Google Scholar
Keggin JF. The structure and formula of 12-phosphotungstic acid. Proceedings of the Royal Society A. Mathematical, Physical and Engineering Sciences. 1934;144(851):75–100. doi: https://doi.org/10.1098/rspa.1934.0035. Google Scholar
Geneste G, Morillo J, Finocchi F. Adsorption and diffusion of Mg, O, and O2 on the MgO(001) flat surface. The Journal of Chemical Physics. 2005;122(17):174707. doi: https://doi.org/10.1063/1.1886734. Google Scholar
Idriss H, Barteau MA, Active sites on oxides: from single crystals to catalysts. Advances in Catalysis. 2000;45:261–331. doi: https://doi.org/10.1016/S0360-0564(02)45016-X. Google Scholar
Freund H-J, Pacchioni G. Oxide ultra-thin films on metals: new materials for the design of supported metal catalysts. Chemical Society Reviews. 2008;37(10):2224–2242. doi: https://doi.org/10.1039/B718768H. Google Scholar
Pacchioni G. Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Physical Chemistry Chemical Physics. 2013;15(6):1737–1757. doi: https://doi.org/10.1039/C2CP43731G. Google Scholar
Natorbs (version 3.0) – universal tool for computing natural (spin)orbitals and natural orbitals for chemical valence http://www.chemia.uj.edu.pl/~mradon/natorbs. Google Scholar
Nalewajski RF, Mrozek J, Formosinho SJ, Varandas AJC. Quantum mechanical valence study of a bond-breaking–bond-forming process in triatomic systems. International Journal of Quantum Chemistry. 1994;52(5):1153–1176. doi: https://doi.org/10.1002/qua.560520504. Google Scholar
Nalewajski RF, Mrozek J. Modified valence indices from the two-particle density matrix. International Journal of Quantum Chemistry. 1994;51(4):187-200. doi: https://doi.org/10.1002/qua.560510403. Google Scholar
Nalewajski RF, Mrozek J, Mazur G. Quantum chemical valence indices from the one-determinantal difference approach. Canadian Journal of Chemistry. 1996;74(6):1121–1130. doi: https://doi.org/10.1139/v96-126. Google Scholar
Nalewajski RF, Mrozek J, Michalak A. Two-electron valence indices from the Kohn-Sham orbitals. International Journal of Quantum Chemistry. 1997;61(3):589–601. doi: https://doi.org/10.1002/(SICI)1097-461X(1997)61:3<589::AID-QUA28>3.0.CO;2-2. Google Scholar
Nalewajski RF, Mrozek J, Michalak A. Exploring bonding patterns of molecular systems using quantum mechanical bond multiplicities. Polish Journal of Chemistry. 1998;72(2S):1779–1791. Google Scholar
Mitoraj M, Michalak A. Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. Journal of Molecular Modeling. 2007;13(2):347–355. doi: https://doi.org/10.1007/s00894-006-0149-4. Google Scholar
Reed AE, Weinstock RB, Weinhold F. Natural population analysis. The Journal of Chemical Physics. 1985;83(2):735. doi: https://doi.org/10.1063/1.449486. Google Scholar
Mayer I. Charge, bond order and valence in the AB initio SCF theory. Chemical Physics Letters. 1983;97(3):270–274. doi: https://doi.org/10.1016/0009-614(83)80005-0. Google Scholar
Bridgeman AJ, Cavigliasso G, Ireland LR, Rothery J. The Mayer bond order as a tool in inorganic chemistry. Journal of the Chemical Society, Dalton Transactions. 2001;14: 2095–2108. doi: https://doi.org/10.1039/B102094N. Google Scholar
Fowe EP, Therrien B, Süss-Fink G, Daul C. Electron-structure calculations and bond order analysis using density functional theory of cationic dinuclear arene ruthenium complexes. Inorganic Chemistry. 2008;47(1):42–48. doi: https://doi.org/10.1021/ic7007914. Google Scholar
Klamt A, Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. Journal of Chemical Society, Perkin Transactions 2. 1993;5:799–805. doi: https://doi.org/10.1039/P29930000799. Google Scholar
Pobrania
Opublikowane
Jak cytować
Numer
Dział
Licencja
Prawa autorskie (c) 2020 Państwowa Wyższa Szkoła Zawodowa w Tarnowie & Autor

Utwór dostępny jest na licencji Creative Commons Uznanie autorstwa – Użycie niekomercyjne 4.0 Międzynarodowe.



