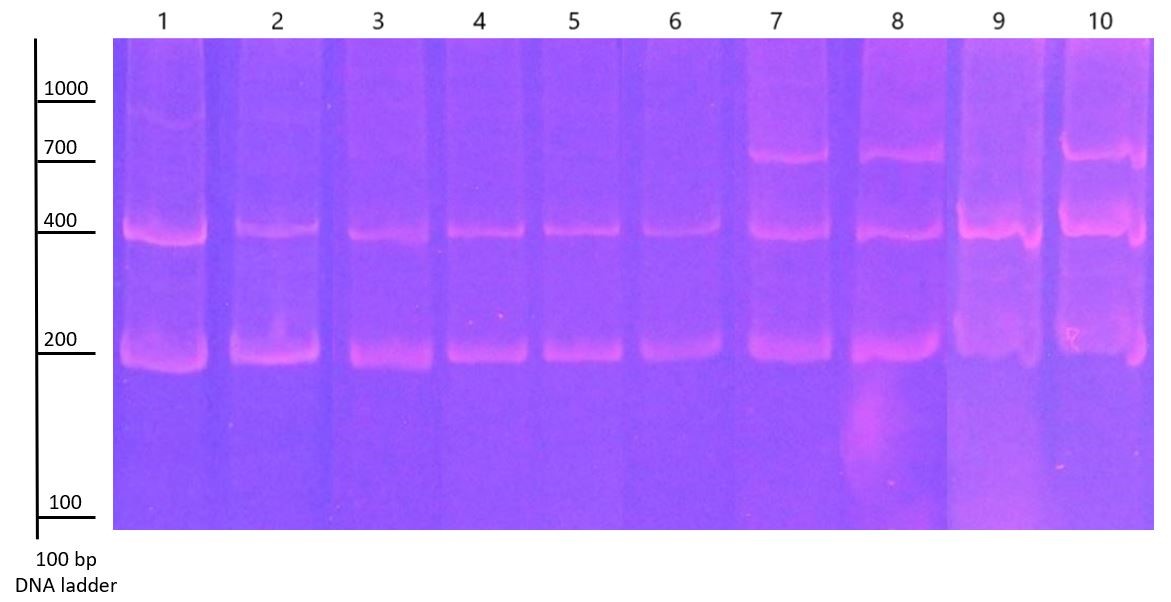
BBAP amplification profiles of apple varieties
DOI:
https://doi.org/10.5604/01.3001.0015.5233Keywords:
Bet v 1, allergens, degenerate primers, apple varietiesAbstract
Several types of allergies are currently known and are characterized by an exaggerated response of the immune system to substances from various sources called allergens. One of them is food allergy, which is becoming more common in the population. For this reason, it is necessary to describe the issue from several aspects including genomic variability of plant allergens. The objective of this study was to analyse intraspecific variability of Bet v 1 of 10 different varieties of apple species (Malus domestica Borkh.). BBAP technique for genomic determination of the presence of Bet v 1 homologs at the DNA level was performed. Degenerate primers that anneal a variable and conserved part of PR-10 protein homologues genes were used in the analyse. Amplicons were generated and formed relatively monomorphic profiles, indicating the stability of the given isoforms of Bet v 1 within the selected apple varieties. To evaluate the potential allergenicity of selected varieties further studies on another molecular level such as a comparison of gene expression of the PR-10 family members and their protein expression levels are needed.
Downloads
References
Phipps JB, Robertson KR, Smith PG, Rohrer JR. A checklist of the subfamily Maloideae (Rosaceae). Canadian Journal of Botany. 1990;68(10):2209–2269. doi: https://doi.org/10.1139/b90-288. Google Scholar
Janick J, Moore JN, editors. Fruit breeding, tree and tropical fruits. New York: John Wiley & Sons; 1996. p. 1–77. Google Scholar
Food and Agriculture Organization of the United Nations (FAO). [Internet] 2019. Available from: http://www.fao.org. Google Scholar
Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutrition Journal. 2004;3:5. doi: https://doi.org/10.1186/1475-2891-3-5. Google Scholar
Somssich IE, Schmelzer E, Kawalleck P, Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Molecular and General Genetics. 1988;213:93–98. doi: https://doi.org/10.1007/BF00333403. Google Scholar
Fernandes H, Michalska K, Sikorski M, Jaskolski M. Structural and functional aspects of PR-10 proteins. The FEBS Journal. 2013;280:1169–1199. doi: https://doi.org/10.1111/febs.12114. Google Scholar
Matthes A, Schmitz-Eiberger M. Apple (Malus domestica L. Borkh.) allergen Mal d 1: effect of cultivar, cultivation system, and storage conditions. Journal of Agricultural and Food Chemistry. 2009;57:10548–10553. doi: https://doi.org/10.1021/jf901938q. Google Scholar
Son DY, Scheurer S, Hoffmann A, Haustein D, Vieths S. Pollen-related food allergy: cloning and immunological analysis of isoforms and mutants of Mal d 1, the major apple allergen, and Bet v 1, the major birch pollen allergen. European Journal of Nutrition. 1999;38:201–215. doi: https://doi.org/10.1007/s003940050063. Google Scholar
Moraes AH, Asam C, Almeida FCL, Wallner M, Ferreira F, Valente AP. Structural basis for cross-reactivity and conformation fluctuation of the major beech pollen allergen Fag s 1. Scientific Reports. 2018;8(1):1–10. doi: https://doi.org/10.1038/s41598-018-28358-1. Google Scholar
Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. The Journal of Allergy and Clinical Immunology. 2000;106(1):27–36. doi: https://doi.org/10.1067/mai.2000.106929. Google Scholar
Varshney RK, Graner A, Sorrells ME. Genic molecular markers in plants: development and applications. Genomics-Assisted Crop Improvement: vol. 1: Genomics Approaches and Platforms. Dordrecht: Springer; 2007. p. 13–29. Google Scholar
Biedermann T, Winther L, Till SJ, Panzner P, Knulst A, Valovirta E. Birch pollen allergy in Europe. Allergy. 2019; 74:1237–1248. doi: https://doi.org/10.1016/j.tibtech.2004.11.005. Google Scholar
Ballmer-Weber BK. Food allergy in adolescence and adulthood. Chemical Immunology and Allergy. 2015;101:51–58. doi: https://doi.org/10.1159/000371669. Google Scholar
Fu L, Cherayil BJ, Shi H, Wang Y, Zhu Y. Species and structure of food allergens: epitopes and cross-reactivity. In: Food Allergy: From Molecular Mechanisms to Control Strategies. Singapore: Springer; 2019. p. 13–39. Google Scholar
Pastorello EA, Ortolani C. Oral allergy syndrome. In: Metcalfe DD, Sampson HA, Simon RA, editors. Food Allergy: Adverse Reactions to Food and Food Additives. Cambridge: Blackwell Science; 1997. p. 221–234. Google Scholar
Desjardins P, Conklin D. Nanodrop microvolume quantitation of nucleic acids. Journal of Visualised Experiments. 2010;45:2565. doi: https://doi.org/10.3791/2565. Google Scholar
White TJ et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols a guide to methods and applications. San Diego: Academic Press; 1990. p. 315–322. Google Scholar
Žiarovská J, Zeleňáková L. Central and Eastern European spring pollen allergens and their expression analysis-state of the art. Diversity. 2016;8(4):1–11. doi: https://doi.org/10.3390/d8040019. Google Scholar
Urbanová L, Žiarovská J. Variability of DNA based amplicon profiles generated by Bet v 1 homologous among different vegetable species. Acta Fytotechnica et Zootechnica. 2021;24(1):1–6. doi: https://doi.org/10.15414/afz.2021.24.mi-apa.1-6. Google Scholar
Kuras A, Antonius K, Kalendar R, Kruczynska D, Korbin M. Application of five DNA marker techniques to distinguish between five apple (Malus × domestica Borkh.) cultivars and their sports. The Journal of Horticultural Science and Biotechnology. 2013;88(6):790–794. doi: https://doi.org/10.1080/14620316.2013.11513040. Google Scholar
Goulao L, Oliveira CM. Molecular characterisation of cultivars of apple (Malus × domestica Borkh.) using microsatellite (SSR and ISSR) markers. Euphytica. 2001;122:81–89. doi: https://doi.org/10.1023/A:1012691814643. Google Scholar
Bilčíková J, Farkasová S, Žiarovská J. Genetic variability of commercially important apple varieties (Malus × domestica Borkh.) assessed by CDDP markers. Acta Fytotechnica et Zootechnica. 2021;24:21–26. doi: https://doi.org/10.15414/afz.2021.24.mi-apa.21-26. Google Scholar
Kafkas S, Özgen M, Doğan Y, Özcan B, Ercişli S, Serçe S. Molecular characterization of mulberry accessions in Turkey by AFLP markers. Journal of the American Society for Horticultural Science. 2008;4:593–597. doi: https://doi.org/10.21273/JASHS.133.4.593, Google Scholar
Pavlović N, Zdravković J, Cvikić D, Zdravković M, Adžić S, Pavlović S, Šurlan-Momirović G. Characterization of onion genotypes by use of RAPD markers. Genetika. 2012;2:269–278. doi: https://doi.org/10.2298/GENSR1202269P. Google Scholar
Asero R, Marzban G, Martinelli A, Zaccarini M, Laimer da Camara Machado M. Search for low allergenic apple cultivars for birch pollen allergic patients: is there a correlation between in vitro assays and patient response? European Annals of Allergy and Clinical Immunolgy. 2006;38(3):94–98. Google Scholar
Sancho AI, Foxall R, Browne T, Dey R, Zuidmeer L, Marzban G, Waldron KW, van Ree R, Hoffmann-Sommergruber K, Laimer M, Mills EN. Effect of postharvest storage on the expression of the apple allergen Mal d 1. Journal of Agricultural and Food Chemistry. 2006;54(16):5917−23. doi: https://doi.org/10.1021/jf060880m. Google Scholar
Kschonsek J, Dietz A, Wiegand C, Hipler UCh, Böhm V. Allergenicity of apple allergen Mal d 1 as effected by polyphenols and polyphenol oxidase due to enzymatic browning. LWT. 2019;113:108289. doi: https://doi.org/10.1016/j.lwt.2019.108289. Google Scholar
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



