Uptake of plastic microbeads by ciliate Paramecium aurelia
DOI:
https://doi.org/10.5604/01.3001.0014.4173Keywords:
microplastics, particles, ingestion, ciliates, Paramecium aureliaAbstract
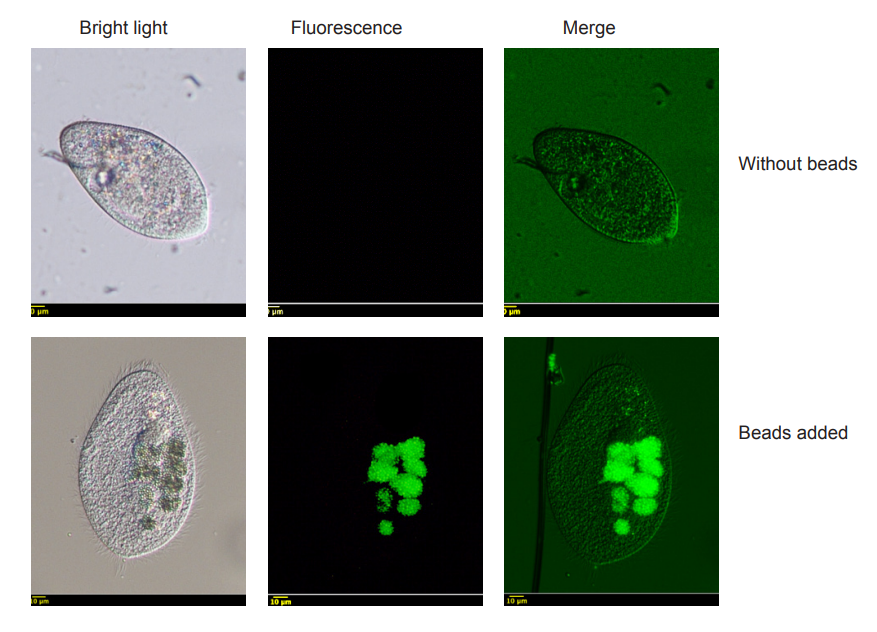
Microplastics (MPs) are small fraction of plastics that are less than 5 mm in length. They are bountiful and widespread pollutants in the aquatic environment. A wide range of organisms which play an important role in the food web, ingest microplastic particles and transfer them to the higher trophic levels. In this work, ingestion of fluorescent polystyrene beads 2 µm of diameter by ciliated protozoa Paramecium aurelia in different concentrations and times of exposure was studied. We studied also the ingestion and clearance rate as well as formation of food vacuoles. The highest uptake of beads by ciliates reached 1047.2 ± 414.46 particles after 10 min of incubation. Food vacuoles formation reflected the ingestion rate of P. aurelia, which increased at higher beads concentration up to the10th minute of incubation and decreased afterwards. On the contrary, the clearance rate persisted to be higher at low concentration. These findings showed that maximum capacity of microplastics ingestion by paramecia depended on beads concentration and on time of exposure.
Downloads
References
Thompson RC, Swan SH, Moore CJ, Vom Saal FS. Our plastic age. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1526):1973–1976. https://doi.org/10.1098/rstb.2009.0054. Google Scholar
PlasticsEurope. Plastics – the facts 2018; 2018:38. Available at: Plastics_the_facts_2018_AF_web.pdf (plasticseurope.org). Google Scholar
Ritchie H, Roser M. Plastic pollution. Our World in Data. 2018;September. Available at: https://ourworldindata.org/plastic-pollution. Google Scholar
Cheung PK, Fok L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Research. 2017;122:53–61. https://doi.org/10.1016/j.watres.2017.05.053. Google Scholar
Lutcavage ME, Plotkin P, Witherington B, Lutz PL. Human impacts on sea turtle survival. In: Lutz PL, Musik JA, editors.The biology of sea turtles. Vol. 1. Boca Raton: CRC Press; 1997. p. 387–409. https://doi.org/10.1201/9780203737088. Google Scholar
Barreiros JP, Barcelos J. Plastic ingestion by a leatherback turtle Dermochelys coriacea from the Azores (NE Atlantic). Marine Pollution Bulletin. 2001;42(11):1196–1197. https://doi.org/10.1016/S0025-326X(01)00215-6. Google Scholar
Moore CJ. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environmental Research. 2008;108(2):131–139. https://doi.org/10.1016/j.envres.2008.07.025. Google Scholar
Wang Y, Zhang D, Zhang M, Mu J, Ding G, Mao Z, Cao Y, Jin F, Cong Y, Wang L, Zhang W, Wang J. Effects of ingested polystyrene microplastics on brine shrimp, Artemia parthenogenetica. Environmental Pollution. 2019;244:715–722. https://doi.org/10.1016/j.envpol.2018.10.024. Google Scholar
Botterell ZLR, Beaumont N, Dorrington T, Steinke M, Thompson RC, Lindeque PK. Bioavailability and effects of microplastics on marine zooplankton: A review. Environmental Pollution. 2019;245:98–110. https://doi.org/10.1016/j.envpol.2018.10.065. Google Scholar
Fry DM, Fefer SI, Sileo L. (1987). Ingestion of plastic debris by Laysan Albatrosses and Wedge-tailed Shearwaters in the Hawaiian Islands. Marine Pollution Bulletin. 1987;18(6 suppl. B):339–343. https://doi.org/10.1016/S0025-326X(87)80022-X. Google Scholar
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. Microplastic ingestion by zooplankton. Environmental Science and Technology. 2013;47(12):6646–6655. https://doi.org/10.1021/es400663f. Google Scholar
Vroom RJE, Koelmans AA, Besseling E, Halsband C. Aging of microplastics promotes their ingestion by marine zooplankton. Environmental Pollution. 2017;231:987–996. https://doi.org/10.1016/j.envpol.2017.08.088. Google Scholar
-Medrano D, Thompson RC, Aldridge DC. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Research. 2015;75:63–82. https://doi.org/10.1016/j.watres.2015.02.012. Google Scholar
Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environmental Pollution. 2013;178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031. Google Scholar
Sharma S, Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environmental Science and Pollution Research. 2017;24(27):21530–21547. https://doi.org/10.1007/s11356-017-9910-8. Google Scholar
de Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN. Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Science of the Total Environment. 2018;645:1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207. Google Scholar
Peters RH. Methods for the study of feeding, grazing and assimilation by zooplankton. In: Downing JA, Rigler FH, editors. A Manual on methods for the assessment of secondary productivity in fresh waters. 2nd ed. Ocford: Blackwell Scientific; 1984. p. 336–412. Google Scholar
Fok AK, Sison BC, Ueno MS, Allen RD. Phagosome formation in Paramecium: effects of solid particles. Journal of Cell Science. 1988;90(Pt 3):517–524. Google Scholar
Berger JD, Pollock C. Kinetics of food vacuole accumulation and loss in Paramecium tetraurelia. Transactions of the American Microscopical Society. 1981;100(2):120–133. Google Scholar
Ramoino P. Changes in the rate of food vacuole formation during early clonal life of paramecium primaurelia. Bolletino Di Zoologia. 1993;60(2):143–146. https://doi.org/10.1080/11250009309355803. Google Scholar
Ali TH, Saleh DS. A simplified experimental model for clearance of some pathogenic bacteria using common bacterivorous ciliated spp. in Tigris river. Applied Water Science. 2014;4(1):63–71. https://doi.org/10.1007/s13201-013-0130-1. Google Scholar
Fenchel T. Suspension feeding in, Ciliated Protozoa: structure and function of feeding organelles. Archiv für Protistenkunde. 1980;123(3):239–260. https://doi.org/10.1016/S0003-9365(80)80009- Google Scholar
Mueller M, Röhlich P, Törö I. Studies on feeding and digestion in Protozoa. VII. Ingestion of polystyrene latex particles and its early effect on Acid Phosphatase in Paramecium multimicronucleatum and Tetrahymena pyriformis. The Journal of Protozoology. 1965;12(1):27–34. https://doi.org/10.1111/j.1550-7408.1965.tb01807.x. Google Scholar
Bragg AN. Selection of Food in Paramecium trichium. Physiological Zoology. 1936;9(4):433–442. http://www.jstor.org/stable/30151388. Google Scholar
Snell TW, Hicks DG. Assessing toxicity of nanoparticles using Brachionus manjavacas (Rotifera). Environmental Toxicology. 2011;26(2):146–152. https://doi.org/10.1002/tox.20538. Google Scholar
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Zhou B, Souissi S, Lee SJ, Lee JS. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the Monogonont Rotifer (Brachionus koreanus). Environmental Science and Technology. 2016;50(16):8849–8857. https://doi.org/10.1021/acs.est.6b01441. Google Scholar
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 University of Applied Sciences in Tarnow, Poland & Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



